The observation of Louis Pasteur (1848) that crystals of certain compounds exist in the form of mirror images laid the foundation of modern stereochemistry. He demonstrated that aqueous solutions of both types of crystals showed optical rotation, equal in magnitude (for solution of equal concentration) but opposite in direction. He believed that this difference in arrangements of atoms (configurations) in two types of crystals. Dutch scientist, J. Van’t Hoff and French scientist, C. Le Bel in the same year (1874), independently argued that the spatial arrangement of four groups (valencies) around a central carbon is tetrahedral and
`color{green}("Asymmetric Carbon" )` : If all the substituents attached to that carbon are different, such a carbon is called asymmetric carbon or stereocentre.
● The resulting molecule would lack symmetry and is referred to as asymmetric molecule.
● The asymmetry of the molecule is responsible for the optical activity in such organic compounds.
`=>` The symmetry and asymmetry are also observed in many day to day objects : a sphere, a cube, a cone are all identical to their mirror images and can be superimposed.
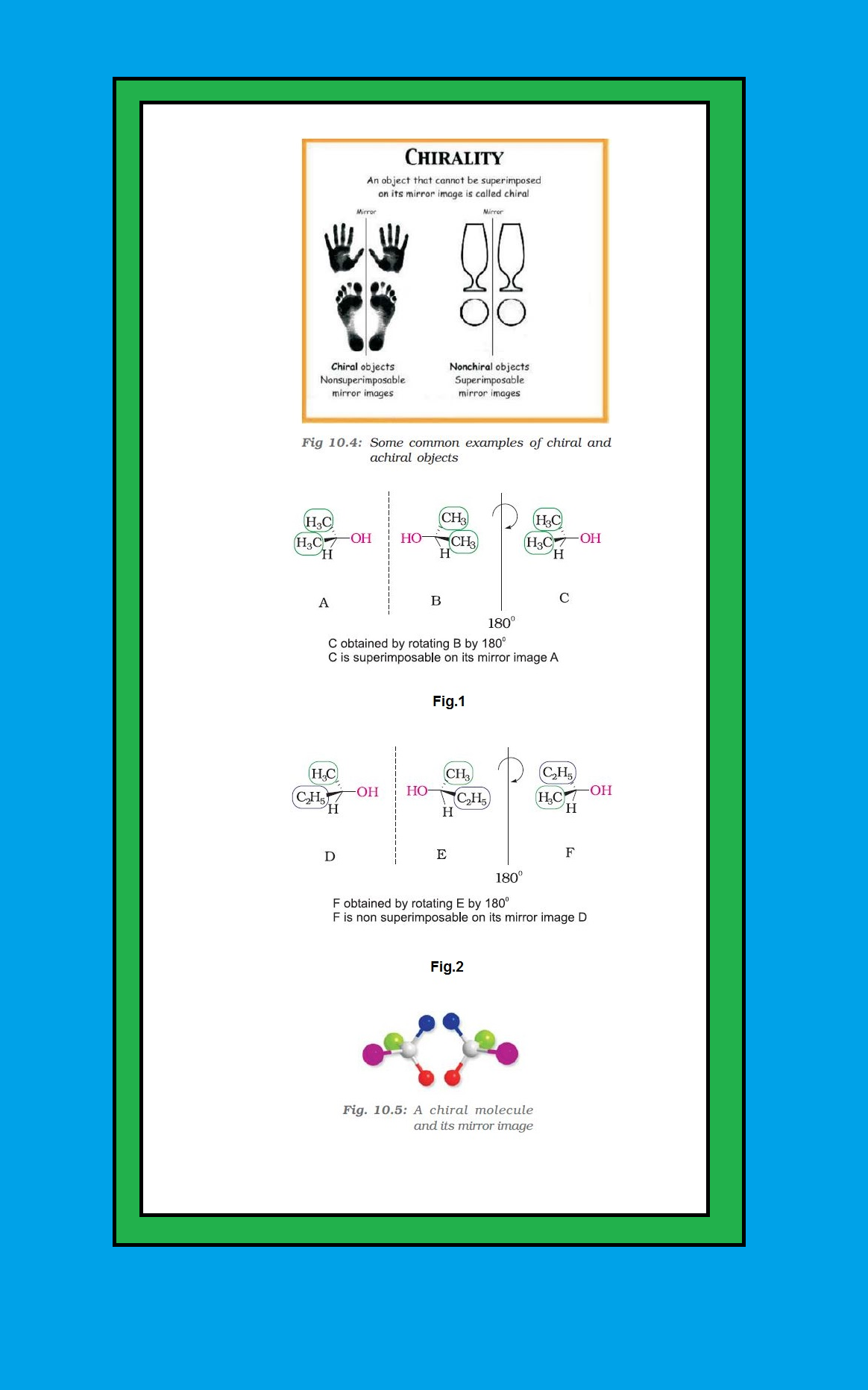
`color{green}("Chirality ")` : The objects which are non-superimposable on their mirror image (like a pair of hands) are said to be `color{green}("chiral")` and this property is known as chirality. For example, your left and right hand look similar but if you put your left hand on your right hand, they do not coincide.
`color{green}("Achiral Objects ")` : The objects, which are, superimposable on their mirror images are called achiral.
`=>` The above test of molecular chirality can be applied to organic molecules by constructing models and its mirror images or by drawing three dimensional structures and attempting to superimpose them in our minds. The aids that can assist us in recognising chiral molecules is the presence of a single asymmetric carbon atom.
● Let us consider two simple molecules propan-2-ol and butan-2-ol and their mirror images.
● Propan-2-ol does not contain an asymmetric carbon, as all the four groups attached to the tetrahedral carbon are not different. Thus it is an achiral molecule. See fig.1.
● Butan-2-ol has four different groups attached to the tetrahedral carbon and as expected is chiral. See fig.2.
● Some common examples of chiral molecules such as 2-chlorobutane, 2, 3-dihyroxypropanal, `color{red}((OHC–CHOH–CH_2OH))`, bromochloro-iodomethane `color{red}((BrClCHI))`, 2-bromopropanoic acid `color{red}((H_3C–CHBr–COOH))`, etc.
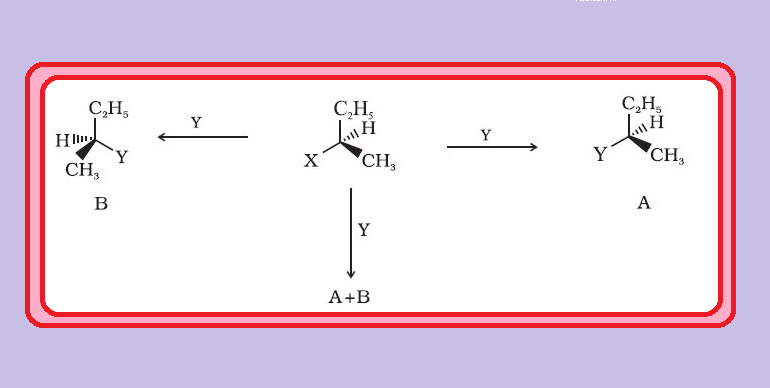
`color{green}("Enantiomers ")` : The stereoisomers related to each other as nonsuperimposable mirror images are called enantiomers (Fig. 10.5).
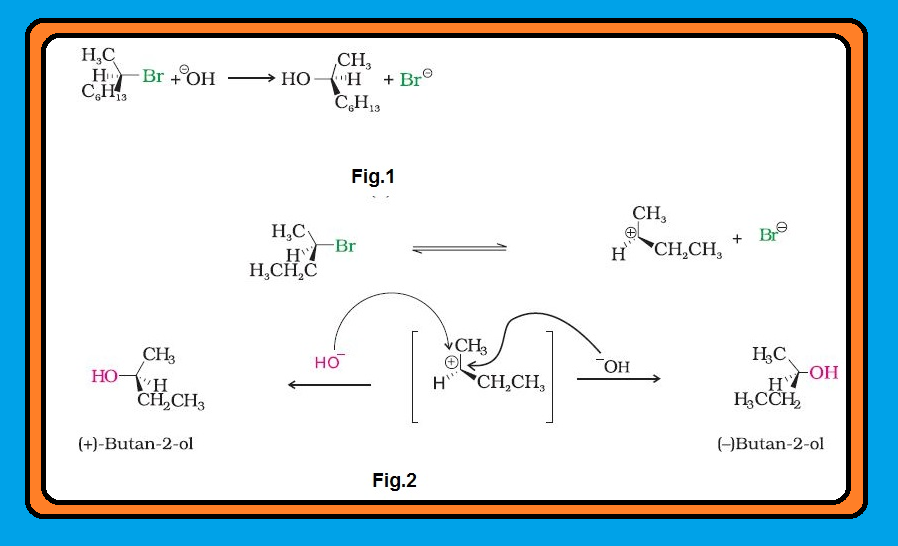
● Enantiomers possess identical physical properties namely, melting point, boiling point, solubility, refractive index, etc.
● They only differ with respect to the rotation of plane polarised light. If one of the enantiomer is dextro rotatory, the other will be laevo rotatory.
`color{green}("Racemic Mixture ") ` : A mixture containing two enantiomers in equal proportions will have zero optical rotation, as the rotation due to one isomer will be cancelled by the rotation due to the other isomer. Such a mixture is known as racemic mixture or racemic modification.
● A racemic mixture is represented by prefixing `color{red}(dl)` or (`color{red}(±)`) before the name, for example (`color{red}(±)`) butan-2-ol.
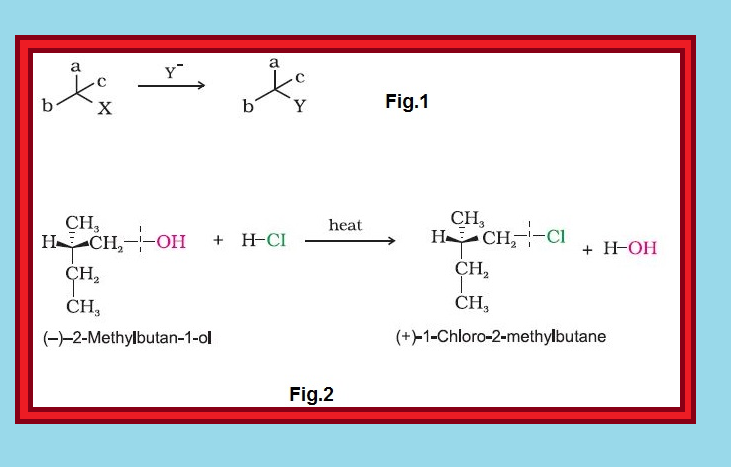
● The process of conversion of enantiomer into a racemic mixture is known as `text(racemisation)`.
The observation of Louis Pasteur (1848) that crystals of certain compounds exist in the form of mirror images laid the foundation of modern stereochemistry. He demonstrated that aqueous solutions of both types of crystals showed optical rotation, equal in magnitude (for solution of equal concentration) but opposite in direction. He believed that this difference in arrangements of atoms (configurations) in two types of crystals. Dutch scientist, J. Van’t Hoff and French scientist, C. Le Bel in the same year (1874), independently argued that the spatial arrangement of four groups (valencies) around a central carbon is tetrahedral and
`color{green}("Asymmetric Carbon" )` : If all the substituents attached to that carbon are different, such a carbon is called asymmetric carbon or stereocentre.
● The resulting molecule would lack symmetry and is referred to as asymmetric molecule.
● The asymmetry of the molecule is responsible for the optical activity in such organic compounds.
`=>` The symmetry and asymmetry are also observed in many day to day objects : a sphere, a cube, a cone are all identical to their mirror images and can be superimposed.
`color{green}("Chirality ")` : The objects which are non-superimposable on their mirror image (like a pair of hands) are said to be `color{green}("chiral")` and this property is known as chirality. For example, your left and right hand look similar but if you put your left hand on your right hand, they do not coincide.
`color{green}("Achiral Objects ")` : The objects, which are, superimposable on their mirror images are called achiral.
`=>` The above test of molecular chirality can be applied to organic molecules by constructing models and its mirror images or by drawing three dimensional structures and attempting to superimpose them in our minds. The aids that can assist us in recognising chiral molecules is the presence of a single asymmetric carbon atom.
● Let us consider two simple molecules propan-2-ol and butan-2-ol and their mirror images.
● Propan-2-ol does not contain an asymmetric carbon, as all the four groups attached to the tetrahedral carbon are not different. Thus it is an achiral molecule. See fig.1.
● Butan-2-ol has four different groups attached to the tetrahedral carbon and as expected is chiral. See fig.2.
● Some common examples of chiral molecules such as 2-chlorobutane, 2, 3-dihyroxypropanal, `color{red}((OHC–CHOH–CH_2OH))`, bromochloro-iodomethane `color{red}((BrClCHI))`, 2-bromopropanoic acid `color{red}((H_3C–CHBr–COOH))`, etc.
`color{green}("Enantiomers ")` : The stereoisomers related to each other as nonsuperimposable mirror images are called enantiomers (Fig. 10.5).
● Enantiomers possess identical physical properties namely, melting point, boiling point, solubility, refractive index, etc.
● They only differ with respect to the rotation of plane polarised light. If one of the enantiomer is dextro rotatory, the other will be laevo rotatory.
`color{green}("Racemic Mixture ") ` : A mixture containing two enantiomers in equal proportions will have zero optical rotation, as the rotation due to one isomer will be cancelled by the rotation due to the other isomer. Such a mixture is known as racemic mixture or racemic modification.
● A racemic mixture is represented by prefixing `color{red}(dl)` or (`color{red}(±)`) before the name, for example (`color{red}(±)`) butan-2-ol.
● The process of conversion of enantiomer into a racemic mixture is known as `text(racemisation)`.
 Explain the following terms.
Explain the following terms.










.png)